What exactly does fluoride do for my teeth anyhow?
Patrick McGann
DDS
Discover how fluoride transforms tooth enamel into a stronger form, enhancing resistance to decay and aiding in remineralization, essential for oral health.
October 10, 2024
In the movie “This is Spinal Tap,” a fictional English rock band is followed through their tour of America and the viewer is given an insight into the highs, lows, and head-scratching commentary of the band. In one scene, Nigel, the lead guitarist, is explaining that his amplifiers go up to 11, not 10 like everybody else’s amplifiers. In his words, they’re “one louder.” When the interviewer asks “why don’t you just make 10 a little louder and make that the top number,” Nigel stares at him for a second and explains “These go to 11.”
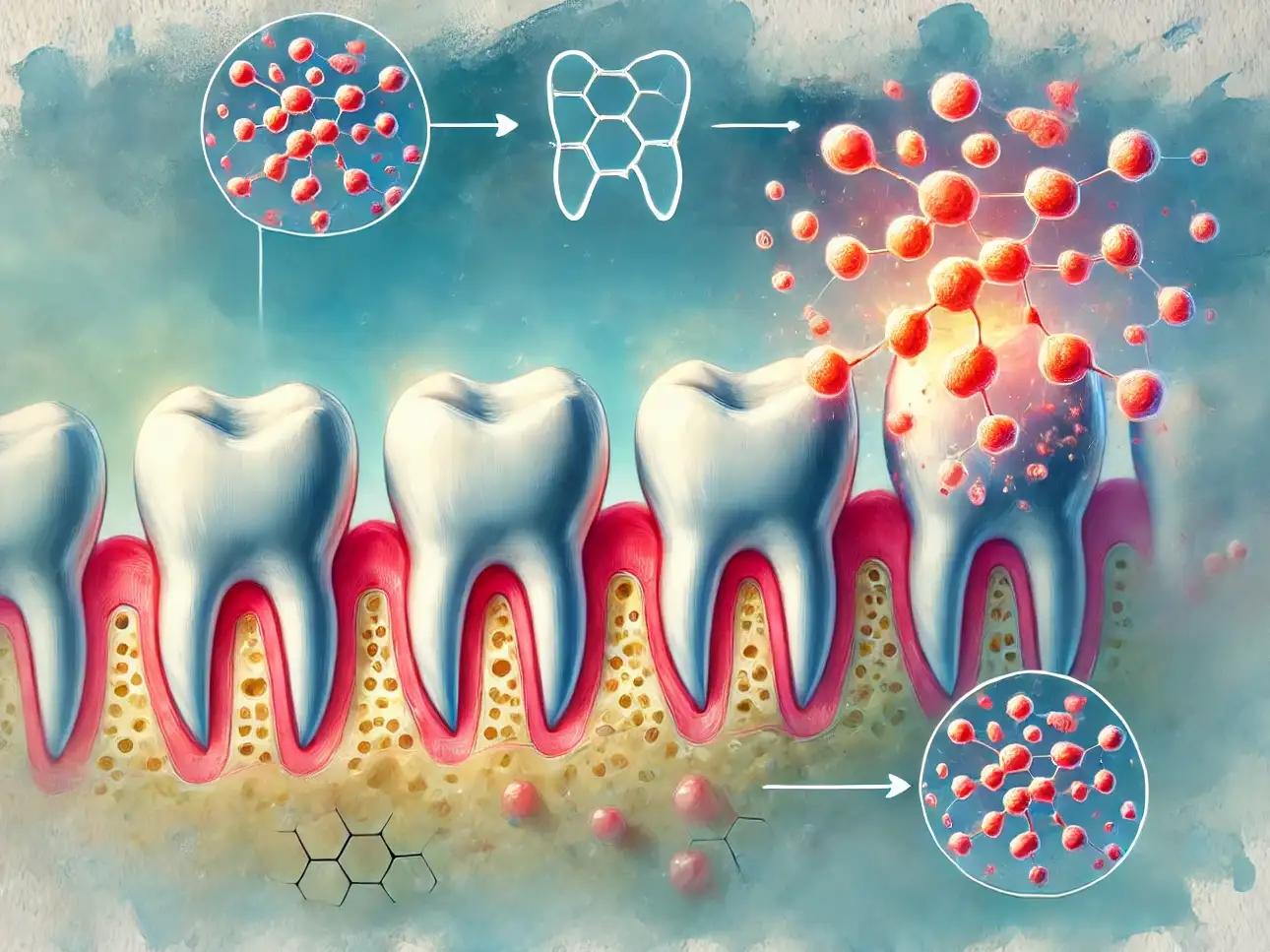
Making an amplifier “one louder” might only require a labeling trick on the dial, and making your teeth “one stronger” may be just as easy to do. Normal tooth structure is composed of a mineral called hydroxyapatite (pronounced like hydroxy-appetite). Calcium and phosphorus make up the “apatite” portion, and a single hydrogen and oxygen make up the “hydroxy” portion. Hydroxyapatite is good at resisting acids, but not great. How do we make it better? Add fluoride! A single fluoride atom comes in, bumps off the hydroxy portion, bonds to the apatite portion, and now you have fluoroapatite, or simply fluorapatite.
With the fluoride now added to your enamel, your teeth are now ‘one stronger,’ at least on the pH scale. Regular hydroxyapatite starts to dissolve at a pH of 5.5, whereas fluorapatite dissolves at a pH of 4.5 (remember that a lower pH means more acidic). However, since the pH scale is not linear, but logarithmic, like the Richter scale for measuring earthquakes, a one-point change from 5.5 to 4.5 actually represents a tenfold increase in resistance to acid attack.
How significant is this? Let’s let millions of years of geology be our guide. It turns out both hydroxyapatite and fluorapatite occur in nature, as components of different types of rock. While hydroxyapatite is quite rare in nature, fluorapatite is rather common. Since one is formed from the other, it stands to reason that this conversion process is the key to withstanding the destructive forces of mother nature.
Certainly the inside of your mouth can’t compare to the forces of nature, but there are similarities. Heat, pressure, abrasives, acids and bacteria, all present to break down whatever is in their path. While smaller in scale and compressed in time frame, your mouth is no less unforgiving than the natural environment.
The Centers for Disease Control and Prevention considers public water fluoridation as one of the ten greatest public health achievements of the 20th century, right up there with immunizations, food safety, and reduction in tobacco use. The CDC notes that fluoride can also slow down the activity of the bacteria in your mouth, and recent evidence shows that it can actually help teeth heal after a small cavity has started. This is a process called remineralization and is the primary benefit of fluoride exposure after age eight.
Fluoride is not without its detractors, and certainly too much fluoride can lead to various health problems. But at normal therapeutic levels, the benefits of fluoride are without question and the negatives are simply without scientific support. So take a tip from Nigel and make your teeth ‘one stronger.’ With the ubiquitous nature of sugar in today’s foods, habits of clenching and grinding being commonplace, and the costs of dental care continuing to rise, our teeth need all the help they can get.
